Medical laminar flow device
A new patented system that offers high ASEPSIA levels
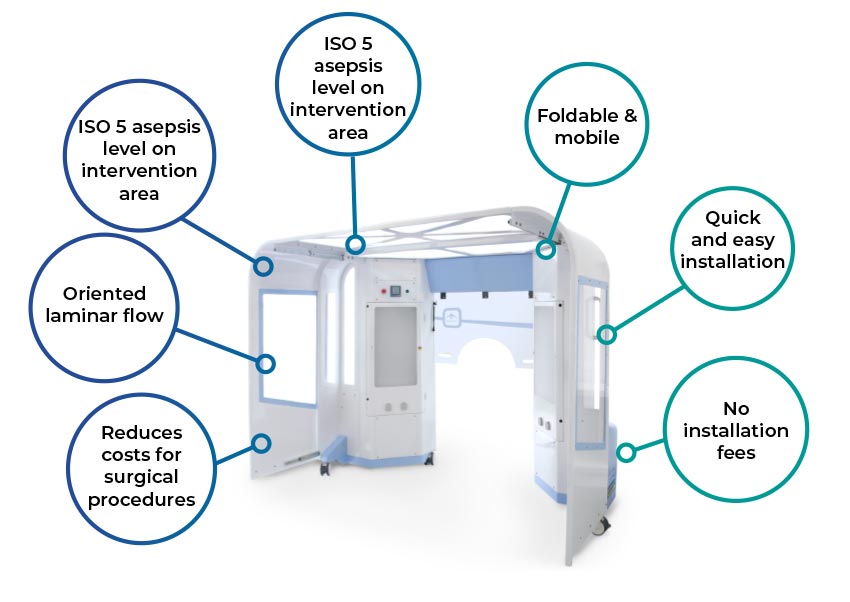
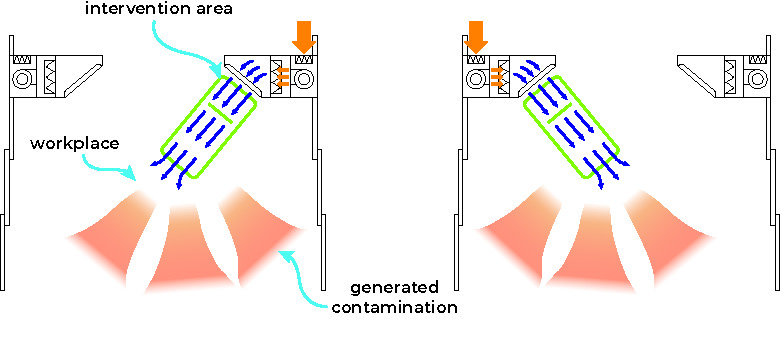
Horizontal oriented flow directly reaching the intervention area
Mobile and retractable (telescopic): You can move and re-locate easily.
Allows to extend the benefits and increases healthcare capacity.
Medical laminar flow device
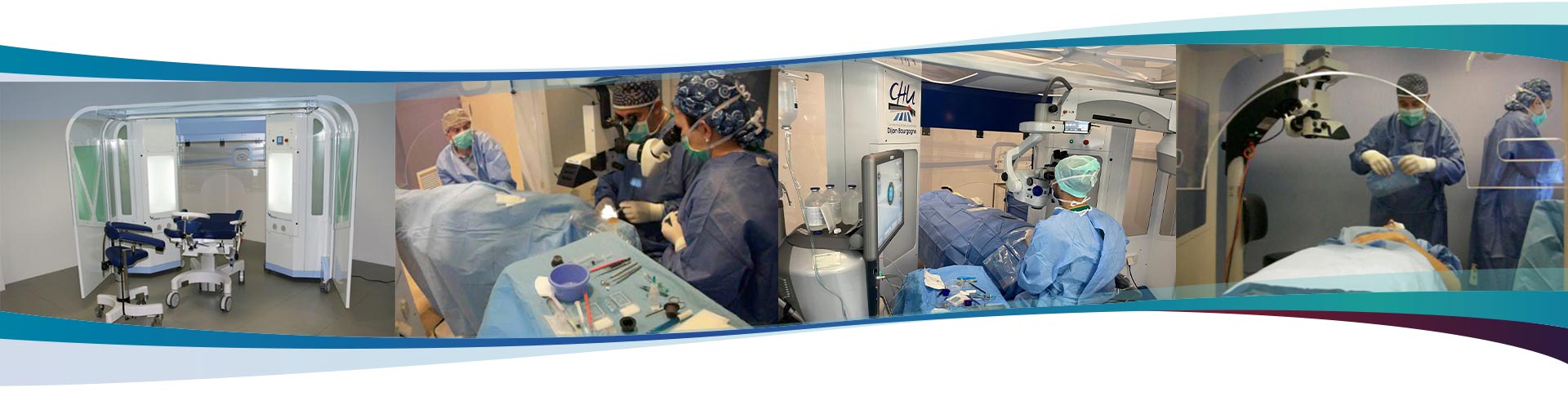
Arc Technologie is an innovative surgical cabin that allows the performance of many surgical procedures, for different specialities. This equipment makes a difference in the healthcare assistance.
ArcSterile allows you to achieve the treatment in a high level of asepsis and with electrical safety conditions for the patient.
Laminar flow
This cabin includes two air impulse columns and air filtering, which generate a tunnel of sterile laminar air flow. This air flow crosses the operative field, sweeping away the micro-particles found in the environment and preventing them from landing on the surgical wound, reducing therefore the risk of infection.
- Aluminum structure, folding walls and front opening door for patient entry.
- Two columns, with two stages of filtration (G3 and HEPA ef. 99.9995%), generates sterile horizontal laminar flow.
- Dual-stage air filter recirculation (G3, H14). Filtering the room´s air, again and again, creating a space of asepsis, independent from time.
- Electronically controlled air speed and pre-set alarms for filter saturation.
- Modules available to facilitate workflow and meet electrical safety requirements.
- Availability for optional modules (biological control, computer systems, central video, surgical lamp, etc.).
- Laminar flow of high-quality air at the site of intervention. ISO 6(open), ISO 5 (closed).
- Low power consumption and simplified cleaning and maintenance.
- Limit the use of chirurgical supplies (surgical drape, sheet)
Data sheets
Declaration of conformity
• 2004/108/CE «Electromagnetic Compatibility Directive» :
EN 50081/2
• 2006/95/CE
«Electrical Safety Directive» :
EN 60204/1, 61010/1
• CEI EN60825 1
«Safety of Laser Products»
• 98/37/CE
«Machinery Safety Directive» :
UNE-EN ISO 12100-1, UNE-EN ISO 12100-2, EN 294, EN 418, EN 349